🔬 Exploring Experimental Cell Therapies Abroad: Legal Status and Costs (2026)
Welcome to WMEDTOUR.com. This in-depth guide is essential for anyone Exploring Experimental Cell Therapies Abroad in 2026. We provide an authoritative, yet compassionate, look at the global landscape, covering crucial legal frameworks and expected costs.
✨ Executive Summary: Key Takeaways
- The market for Exploring Experimental Cell Therapies Abroad is rapidly expanding, driven by patient demand for early access to advanced medicine.
- Legal Status is highly fractured: what is a clinical trial in one nation may be an unregulated procedure in another. Verification is paramount.
- Costs vary dramatically, ranging from $10,000 to over $500,000, depending on the country, complexity, and type of cellular product.
- Leading medical tourism destinations, including Iran, are becoming key centers for specialized, affordable, and quality-controlled experimental therapies.
- Patients must prioritize informed consent and recognize that ‘experimental’ means efficacy is not guaranteed.
⚖️ The Fragmented Global Legal Status of Cell Therapies in 2026
The regulatory environment surrounding cell therapies remains complex and inconsistent globally. Consequently, this fragmentation is the main reason why patients are forced into medical travel. Furthermore, some countries maintain extremely strict, centralized approval processes, while others have adopted more flexible, albeit potentially riskier, models. Therefore, understanding this legal maze is your first step.
The Regulatory Spectrum: Strict vs. Permissive Nations
Major Western nations (USA, EU, Japan) typically operate under two strict pathways for experimental treatments. First, there is the formal Clinical Trial pathway, which requires rigorous phases, often limiting access to specific criteria and locations. Second, there is the Compassionate Use or Expanded Access program, which allows seriously ill patients to use an unapproved product outside a trial when no other options exist. Nevertheless, these paths are often slow and restrictive.
Conversely, many medical tourism hubs adopt more permissive legislation. These regulatory environments can often accelerate patient access. However, this flexibility also carries risk. Specifically, patients must ensure they are receiving treatment within an ethically governed, government-sanctioned framework and not an unregulated commercial venture.
🌐 Case Study: Iran’s Approach to Advanced Experimental Cell Therapies
When considering countries that balance accessibility with governmental oversight, Iran frequently stands out. Iran has strategically positioned itself as a regional hub for specialized medicine, including cell therapy research and application. Consequently, the government has invested heavily in institutions dedicated to advanced research, offering a streamlined process for certain experimental treatments that adhere to national guidelines. Moreover, this approach allows for more affordable access compared to Europe or North America, while still operating within a recognized national health framework. For example, procedures like advanced immunotherapy or specialized stem cell applications are often more accessible here, provided the clinical team follows established ethical and governmental protocols for experimental use. Lung cancer treatment in Iran often incorporates such innovative approaches.
✅ Pros of Iran’s System for Experimental Cell Therapies
- Affordability: Significantly lower costs than Western countries.
- Specialization: High concentration of specialized facilities, particularly in fields like oncology and hematology.
- Accessibility: Often quicker access to treatments under approved protocols.
- Quality Infrastructure: Commitment to modern medical infrastructure and technology.
❌ Cons and Challenges
- Verification: Patients must meticulously verify the specific experimental protocol and the clinic’s credentials.
- Cultural Differences: Navigating a new culture and language requires professional support.
- Follow-Up: Coordinating post-treatment follow-up with home country doctors can be complex.
💰 The Cost Landscape of Experimental Cell Therapies in 2026
The financial commitment for Exploring Experimental Cell Therapies Abroad is arguably the most significant barrier. Indeed, costs fluctuate massively based on three primary factors: the type of cell product, the country of treatment, and whether the procedure is part of a non-funded trial or a fully commercial offering. Generally speaking, insurance coverage is nearly nonexistent for treatments explicitly labeled as ‘experimental’.
Understanding the Cost Drivers
Autologous vs. Allogeneic Therapies
The cellular source heavily influences the price. Autologous therapies use the patient’s own cells (e.g., collecting T-cells, engineering them, and re-infusing them), leading to high processing and manufacturing costs. These therapies, such as customized CAR-T for cancer, easily reach into the hundreds of thousands of dollars globally, even when experimental. Conversely, Allogeneic therapies use donor cells. While manufacturing scale-up can potentially lower costs over time, the initial research, donor matching, and regulatory hurdles keep the price high.
Geographic Price Disparities
Geographical location creates enormous price differences. Furthermore, a simple stem cell injection might cost $5,000 in a lower-cost country but over $30,000 in a leading Western European clinic. For example, complex cellular immunotherapy for oncology, such as advanced T-cell therapies (TCR-T or TIL therapy), could cost $400,000 in the US versus potentially $100,000 to $250,000 in an established medical tourism destination like Turkey or Iran, provided the treatment falls under a structured program. The lower cost of labor, facility overhead, and a different research funding model allow for these price reductions, as seen in the general cancer treatment cost comparisons.
Cost Comparison Table: Key Regions (2026 Estimates)
The following table provides a broad overview of estimated costs for a complex experimental cell therapy (e.g., advanced stem cell treatment for chronic disease or a novel immunotherapy protocol). Prices are highly variable and serve as estimates only.
| Region/Country | Estimated Cost Range (USD) | Key Legal Status Note | Typical Wait Time |
|---|---|---|---|
| United States/EU | $150,000 – $500,000+ | Strictly clinical trials or compassionate use; high regulatory burden. | Long (Trial enrollment required) |
| Iran | $25,000 – $120,000 | Government-supported research and specialized application; strong institutional oversight. | Short to Moderate |
| Turkey | $40,000 – $180,000 | Regulated clinical trials and some specialized, approved protocols; growing innovation sector. | Short |
| East Asia (e.g., South Korea) | $80,000 – $350,000 | Very advanced research but often requires local regulatory approval; high standards. | Moderate |
Consequently, for many patients seeking viable options, 💡 Exploring Experimental Cell Therapies Abroad in countries like Iran or Turkey offers a much-needed financial middle ground.
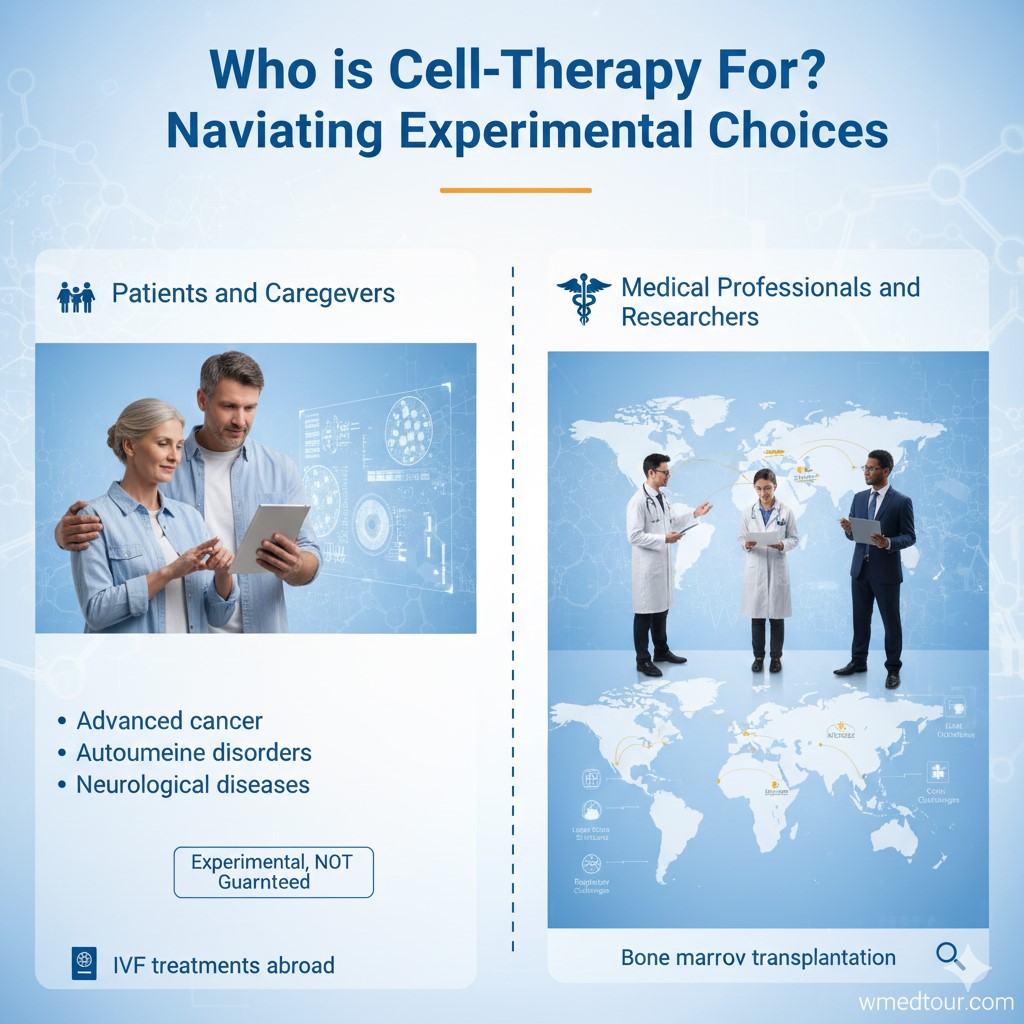
This infographic illustrates the two main audiences involved in cell therapy: Patients and Caregivers (often seeking treatment for advanced cancer, autoimmune, or neurological diseases, which are noted as experimental) and Medical Professionals and Researchers (focusing on global research and procedures like bone marrow transplantation).
🎯 Who is This For? Navigating Experimental Choices
This information is specifically for two groups of individuals.
Patients and Caregivers
Are you or a loved one suffering from a condition that has exhausted standard, approved treatments? Furthermore, are you willing to accept the inherent risks of an unproven therapy? This includes conditions like advanced cancer, certain autoimmune disorders, or degenerative neurological diseases, where cell therapy offers a potential, albeit experimental, lifeline. It is vital to consult with multiple specialists and research facilities before making any decision. Remember that an experimental therapy is not a guaranteed cure, regardless of the claims made. If you are exploring treatments like IVF treatments abroad, you already understand the importance of global medical research.
Medical Professionals and Researchers
Are you a clinician, researcher, or medical tourism professional seeking to understand the global marketplace for cellular medicine? Then this guide offers critical insight into where research protocols are being applied, the typical cost structures, and the regulatory challenges that international patients face. Furthermore, understanding the legal landscape in countries like Iran allows for better professional guidance and ethical referral practices. This knowledge is crucial for coordinating safe and compliant international patient journeys, especially for complex procedures like bone marrow transplantation.
🚧 In-Depth Breakdown: Risks and Benefits
Before committing to Exploring Experimental Cell Therapies Abroad, a clear-eyed assessment of the inherent advantages and disadvantages is mandatory.
Benefits (Pros) 📈
- Potential for Breakthrough: Experimental therapies represent the current edge of medical science and offer hope where conventional treatments have failed.
- Faster Access: In some countries, patients gain access to promising treatments years before they would be approved in their home nation.
- Lower Cost (Selectively): Certain destinations, particularly those focused on medical tourism like Iran and Turkey, offer the treatment at a fraction of the price of the West.
- Advanced Technology: Access to clinics focused purely on research and development often means exposure to the newest technologies and techniques, such as those used in advanced PGT procedures.
Risks (Cons) 🛑
- Efficacy Uncertainty: The treatment is unproven; there is a significant risk it will not work or provide little benefit.
- Safety Concerns: Experimental treatments carry unknown risks, including severe side effects or long-term complications that have not yet been documented.
- Financial Risk: You pay out-of-pocket, and if the treatment fails, you lose a substantial, often life-altering, amount of money.
- Regulatory Void: You risk seeking care in facilities that are minimally regulated, potentially exposing you to exploitation or substandard practice. This highlights the need for due diligence when choosing a clinic.
🗺️ Patient Journey: Seeking Cell Therapy in Iran (Hypothetical Case Study)
Case Profile: Elias, 45, Autoimmune Condition
Elias, a 45-year-old professional from Western Europe, suffered from a debilitating autoimmune condition unresponsive to all approved immunosuppressive drugs. His local specialists offered no further options outside of palliative care.
Phase 1: Research and Vetting 🔎
Elias researched Exploring Experimental Cell Therapies Abroad for his specific condition. He discovered a specialized research institution in Tehran, Iran, running a small, government-approved, expanded-access program using a novel mesenchymal stem cell infusion. His local doctor, while unable to endorse the treatment, reviewed the published research from the Iranian team (published in a reputable, non-competitor university journal) and deemed the protocol scientifically sound, though still experimental. He used resources like WMEDTOUR to understand the local medical scene.
Phase 2: Logistics and Legal Consent 📝
He contacted the institution through a reputable facilitator. Furthermore, the clinic required extensive medical records and a formal consultation. Most importantly, Elias had to sign a rigorous informed consent document explicitly stating that the treatment was experimental and lacked guaranteed success. The cost estimate was $65,000, which was significantly less than the $200,000 quote he received for an equivalent (but unapproved) trial in the US. He secured a medical visa to Iran, relying on the process detailed in the medical travel guides.
Phase 3: Treatment and Outcome 💉
Upon arrival, Elias underwent a thorough check-up. The cell harvesting and infusion process took place over four days. The quality of the facilities was modern and comparable to his expectations. Although the full outcome would take months, he reported no immediate severe side effects. Six months later, Elias reported a significant (50%) reduction in his major symptoms, allowing him to regain much of his mobility and quality of life. The treatment was not a cure, but it offered substantial, life-changing relief.
❓ Frequently Asked Questions (FAQ) about Exploring Experimental Cell Therapies Abroad
General Questions
What defines an ‘experimental’ cell therapy in 2026?
In 2026, an experimental cell therapy is generally defined as one that has not yet received full regulatory approval from major bodies like the FDA, EMA, or equivalent national agencies. Consequently, these treatments are typically still undergoing clinical trials to prove safety and efficacy. Patients seeking these therapies abroad are often accessing these trials or ‘compassionate use’ programs in specific jurisdictions.
Why do patients travel abroad for Exploring Experimental Cell Therapies Abroad?
Patients travel abroad primarily because the therapy they seek is not available, is too expensive, or has not yet been approved in their home country. Some destinations also offer more permissive regulatory environments or faster access to cutting-edge trials. The hope is to gain access to a potentially life-changing treatment sooner.
Is it legal to receive an unapproved experimental cell therapy in any country?
The legality varies significantly by country. Many nations have specific ‘compassionate use’ programs or clinical trial frameworks that allow access under strict conditions. Crucially, what is considered an illegal, unproven therapy in one country might be a legally permissible (though still experimental) procedure in another, particularly in jurisdictions focused on promoting medical tourism and innovation.
How much does Experimental Cell Therapy cost globally in 2026?
The cost is highly variable. Generally, prices range from $10,000 for simpler, autologous (patient’s own cells) procedures in cost-effective regions to well over $500,000 for highly complex, allogeneic (donor cells) therapies like advanced CAR-T treatments in Western nations. Factors like the type of cell, the condition treated, and the country significantly impact the final price.
Does medical insurance cover Experimental Cell Therapies abroad?
Typically, no. Most standard medical insurance policies, both domestic and international, do not cover experimental or unapproved treatments, especially those received in a foreign country outside of a fully accredited, recognized clinical trial that meets the insurer’s criteria. Patients almost always pay out-of-pocket.
What types of cell therapies are most often sought experimentally in 2026?
Commonly sought experimental cell therapies include various forms of stem cell therapy for neurological and orthopedic conditions, advanced immunotherapy (like novel T-cell therapies for cancers not covered by standard CAR-T), and regenerative medicine treatments for conditions like heart failure or diabetes. This area is rapidly evolving, as noted in the research for new cancer treatments.
Legal and Safety Questions
What are the primary legal risks when seeking Experimental Cell Therapies Abroad?
The primary legal risks include lack of legal recourse if the treatment causes harm, exposure to unregulated or uncertified practitioners, and the potential for therapies to be seized or deemed illegal upon returning home. It is absolutely vital to verify the treating facility’s credentials and the country’s specific regulatory framework before travel.
How can I verify the credibility of a foreign clinic offering Experimental Cell Therapy?
You should check for international accreditations (like JCI), look for published peer-reviewed research by the clinicians in question, and verify the institution’s registration with the country’s main health authority. You must also confirm if the treatment is offered as a legitimate clinical trial or an approved compassionate-use protocol, not just a for-profit service. This is similar to vetting a surgeon for complex procedures like robotic surgery.
Are there ethical guidelines for Exploring Experimental Cell Therapies Abroad?
Yes, ethical guidelines emphasize fully informed consent, transparency about the non-proven nature of the therapy, and strict adherence to established protocols, even if the treatment is experimental. Patients must understand that the primary goal is often scientific data collection, not guaranteed cure. Reputable medical tourism facilitators adhere to global standards, such as those governing ethical surgeries.
What is the expected recovery time for an Experimental Cell Therapy?
Recovery time varies drastically based on the specific procedure, the route of cell administration, and the underlying condition. Simple injections might require minimal downtime, while complex bone marrow transplants (a form of cell therapy) require weeks or months of intensive care and follow-up. Patients should always ask for a detailed post-procedure recovery plan.
Do Experimental Cell Therapies have high success rates?
Since these treatments are experimental, proven, high success rates are generally not established. Data is often limited, preliminary, or confined to small case series. Patients must approach these therapies with realistic expectations, understanding that the outcome is uncertain and there is a risk of no benefit or even harm. Success rates are only definitive for approved treatments like certain IVF procedures.
How is the ‘legal status’ of these therapies expected to change by 2026?
By 2026, we anticipate continued global divergence. Major regulatory bodies will likely approve more specific therapies, moving them out of the ‘experimental’ category. Conversely, some countries may tighten regulations to prevent the exploitation of vulnerable patients, while others, like Iran, may continue to streamline access to advanced, specialized treatments to attract medical tourism, as detailed in our guide on Iran’s medical regulations.
🎓 Conclusion: Proceed with Caution and Clarity
Exploring Experimental Cell Therapies Abroad: Legal Status and Costs (2026) offers a path of hope for many patients. However, this journey demands extreme diligence, as the path is fraught with both clinical and legal uncertainty. Consequently, prospective patients must proceed with realistic expectations, prioritize transparency, and partner with reputable medical tourism facilitators like WMEDTOUR who understand the nuances of global cellular medicine and pre-travel logistics. Ultimately, the decision to seek experimental treatment abroad is a deeply personal one that must be grounded in verified facts, not just hope. For further reading on the scientific background of cell therapies, please consult the resources at the National Institutes of Health (NIH) and the International Society for Stem Cell Research (ISSCR).




