Theranostics treatment with Lu177 for metastasis: A Comprehensive Guide to the Future of Targeted Cancer Care
Executive Summary: Key Takeaways on Lu-177 Theranostics
The era of “one-size-fits-all” cancer treatment is steadily concluding, and the advent of Theranostics treatment with Lu177 for metastasis marks a pivotal shift toward personalized medicine. This innovative approach combines both diagnostic imaging and targeted therapy into a single process. Essentially, it allows doctors to first confirm the exact location of metastatic tumors using a radioactive tracer (the ‘thera-‘ part), and then deliver a powerful, tumor-killing radioisotope—Lutetium-177 (Lu-177)—directly to those cells (the ‘-nostics’ part). Lu-177 has fundamentally changed the management landscape for specific, advanced cancers, most notably metastatic castration-resistant prostate cancer (mCRPC) and certain neuroendocrine tumors (NETs). Consequently, patients who previously had limited options are now experiencing improved quality of life and survival rates.
Introduction: The Dawn of Precision Oncology
For decades, treating metastatic cancer—cancer that has spread from its primary site—presented immense challenges. Conventional treatments like chemotherapy and external beam radiation, while effective, often damage healthy tissue alongside cancerous cells, leading to significant side effects. Fortunately, molecular science is rapidly bridging the gap between effective treatment and minimal toxicity. We are now witnessing the revolutionary rise of Theranostics treatment with Lu177 for metastasis.
Theranostics, a portmanteau of “therapy” and “diagnostics,” is a game-changing strategy in oncology. It uses specific molecules to locate and destroy cancer cells while largely sparing healthy surrounding tissue. At the heart of this revolution is Lutetium-177 (Lu-177), a radioisotope known for its ideal therapeutic profile. This comprehensive guide, tailored for both curious individuals and healthcare professionals, dissects the mechanism, applications, benefits, and future promise of this sophisticated treatment.
Before diving into the specifics of Lu-177, it’s helpful to understand the principles of targeted cancer therapy. Unlike traditional approaches, targeted therapy attacks unique features, such as specific receptors or pathways, that exist only on the surface of cancer cells. This specificity is crucial, and it’s what makes Theranostics treatment with Lu177 for metastasis such a powerful tool in the fight against advanced disease.
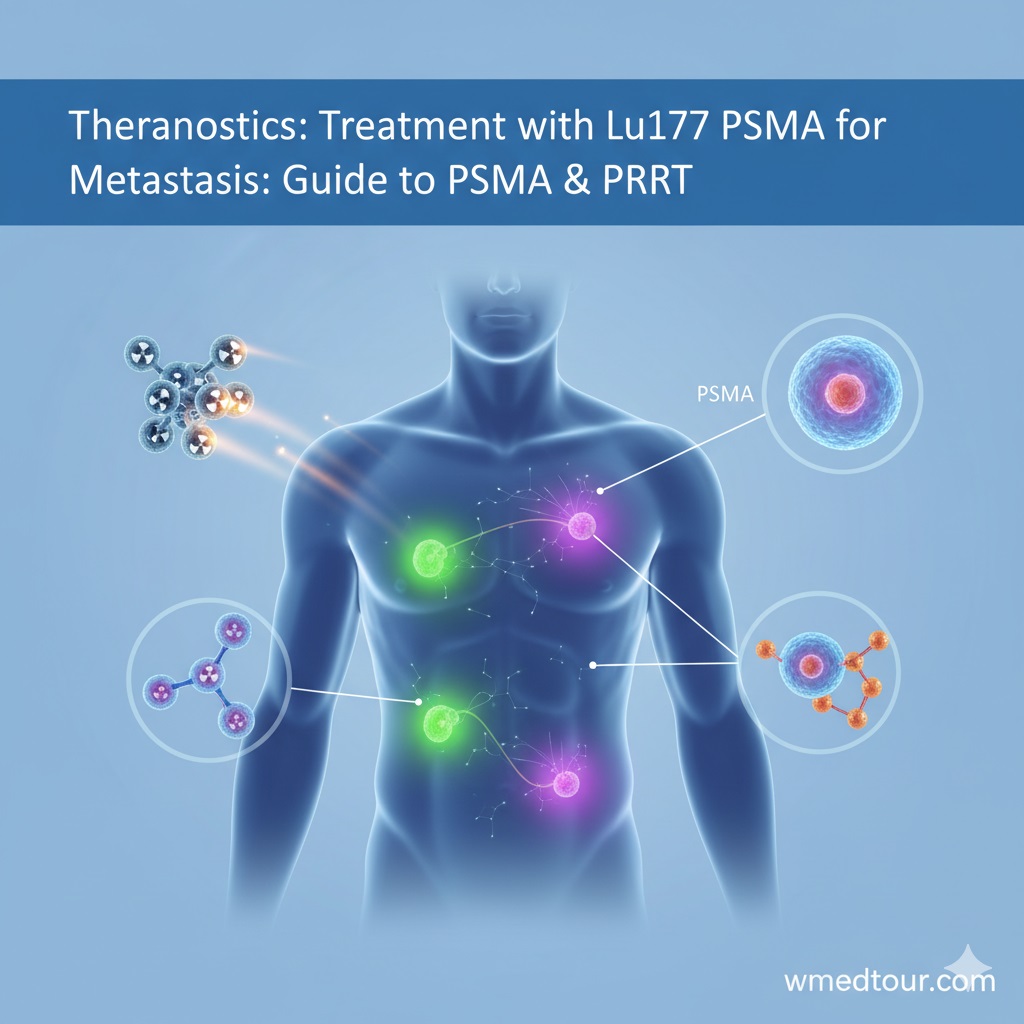
The Science of Theranostics: How Lu-177 Targets Cancer
The success of Lu-177 theranostics hinges on two perfectly matched components: a targeting molecule and a therapeutic radioisotope. Think of this process as a highly specialized missile system where the targeting molecule acts as the guidance system, and Lu-177 serves as the warhead.
The Guidance System: Targeting Molecules (PSMA and DOTATATE)
The targeting molecule is a synthetic peptide or small molecule designed to bind specifically to receptors that are over-expressed on the surface of certain cancer cells. For the most common Lu-177 applications in metastasis, two main targets are utilized:
- PSMA (Prostate-Specific Membrane Antigen): This receptor is found in exceptionally high concentrations on the surface of prostate cancer cells, especially in the metastatic (mCRPC) setting. The diagnostic and therapeutic agents use a molecule that locks onto PSMA.
- SSTR2 (Somatostatin Receptor Subtype 2): These receptors are abundant on the surface of most neuroendocrine tumors (NETs), which can originate in the pancreas, gut, or lung. The targeting agent used here is often called DOTATATE.
The theranostic journey begins with the diagnostic step. A small amount of the targeting molecule is first labelled with a diagnostic radioisotope (like Gallium-68 or Fluorine-18). When injected, this tracer travels through the body, binds to the cancer cells, and emits radiation detectable by a PET scan. This confirms whether the patient’s tumors express the necessary target—PSMA or SSTR2—making them eligible for therapy. Consequently, this diagnostic precision eliminates unnecessary treatment for non-eligible patients.
The Therapeutic Warhead: Lutetium-177 (Lu-177)
Once a patient is deemed a suitable candidate, the same targeting molecule is labeled with the therapeutic radioisotope: Lutetium-177. Lu-177 is ideal because it emits two types of radiation:
- Beta Particles: These are the high-energy, therapeutic particles. They travel a very short distance (typically less than 2mm) within the tissue, meaning they deliver a potent, localized blast of radiation that destroys the cancer cell, yet they spare the neighboring healthy cells.
- Gamma Rays: These are low-energy rays used for post-treatment imaging (SPECT/CT scans). This allows doctors to visualize the treatment’s distribution within the body in real-time, confirming that the radiation reached the target sites successfully.
Therefore, the beauty of the **Theranostics treatment with Lu177 for metastasis** is its ability to simultaneously confirm the target and treat it with ultra-high precision.
Lu-177 PSMA: A Revolution for Metastatic Prostate Cancer
Metastatic Castration-Resistant Prostate Cancer (mCRPC) remains one of the most challenging diseases to manage. When standard hormonal therapies and chemotherapy fail, options become limited. However, the introduction of Lutetium-177-PSMA (177Lu-PSMA) has offered a powerful new line of defense.
The treatment involves multiple cycles, usually administered several weeks apart, allowing time for the body to recover and the next dose to be optimized. This targeted approach has demonstrated remarkable efficacy in clinical trials, leading to significant improvements in overall survival and progression-free survival for many patients. We encourage you to explore other advanced cancer options, such as TCR-T cell receptor therapy, to see the full spectrum of modern oncology.
Pros and Cons of Lu-177 PSMA Therapy
Pros
- High Specificity: PSMA is highly expressed by mCRPC cells, leading to minimal damage to surrounding healthy tissue.
- Improved Survival: Clinical data shows significant prolongation of overall survival compared to standard care in late-stage disease.
- Favorable Side Effect Profile: Side effects are generally milder and more manageable than those associated with systemic chemotherapy.
- Treats Diffuse Metastasis: It can effectively target small, widespread metastatic lesions in lymph nodes, bones, and other organs that are difficult to reach with external radiation.
- Quality of Life: Many patients report reduced pain from bone metastases and improved general well-being.
Cons
- Eligibility: Requires confirmed PSMA expression via a diagnostic PET scan (Ga-68 or F-18 PSMA). Patients without sufficient PSMA expression are ineligible.
- Salivary Gland Toxicity: PSMA is also expressed in the salivary glands, leading to dry mouth (xerostomia) as a common side effect.
- Bone Marrow Suppression: As the radioisotope passes through the bone marrow, it can temporarily reduce white blood cell, red blood cell, and platelet counts.
- Availability: Access remains limited compared to conventional therapies, often requiring travel to specialized oncology departments or centers.
Lu-177 DOTATATE: A Lifeline for Neuroendocrine Tumors (NETs)
Another major success story in precision oncology is the use of Lutetium-177-DOTATATE (177Lu-DOTATATE) for inoperable, advanced, and metastatic neuroendocrine tumors (NETs). These tumors, often slow-growing but potentially lethal, frequently express a high concentration of SSTR2 receptors. Furthermore, this treatment, often referred to as Peptide Receptor Radionuclide Therapy (PRRT), provides a crucial therapeutic option when tumors become resistant to other systemic treatments.
PRRT works similarly to PSMA therapy: the DOTATATE peptide binds to the SSTR2 receptors, and the Lu-177 payload delivers tumoricidal radiation. New methods in cancer treatment are constantly evolving, and PRRT represents one of the most established advanced molecular techniques. For patients with well-differentiated metastatic NETs, PRRT offers durable response rates and improved progression-free survival.
Pros and Cons of Lu-177 DOTATATE (PRRT)
Pros
- Established Efficacy: PRRT is supported by robust clinical trial data showing excellent response rates for metastatic NETs of various origins (e.g., small intestine, pancreas).
- Systemic Control: Achieves control over widespread disease that might otherwise be impossible to treat surgically or with external radiation.
- Targeted Symptom Relief: Highly effective at controlling the hormonal symptoms (carcinoid syndrome) often associated with functional NETs.
- Low Systemic Toxicity: Similar to Lu-177 PSMA, the localized radiation delivery minimizes damage to non-targeted organs.
Cons
- Kidney Protection Required: Because the radiopharmaceutical is primarily cleared through the kidneys, patients must receive a concomitant infusion of specific amino acids to protect renal function.
- Delayed Response: While treatment is effective, the full clinical benefit may take several months to manifest, requiring patience and close monitoring.
- Liver Metastasis Limitation: While effective, large volume liver metastases may require supplementary or alternative local treatments.
- SSTR2 Requirement: Tumors must show adequate SSTR2 expression on a diagnostic scan (Ga-68 DOTATATE PET/CT) to be considered for treatment.
The Patient Journey: A Case Study in Lu-177 PRRT
Case Study: John’s Path to Remission
John, a 72-year-old retired engineer, was diagnosed with advanced, metastatic prostate cancer (mCRPC). After initial hormonal therapy and subsequent chemotherapy failed to control the widespread bone and lymph node metastases, his oncologist recommended investigating Theranostics treatment with Lu177 for metastasis.
Step 1: The Diagnostic Scan. John first underwent a Ga-68 PSMA PET/CT scan. The scan clearly showed high PSMA uptake in all known metastatic sites, confirming his eligibility. He was a perfect candidate.
Step 2: The Treatment Cycle. John received his first dose of 177Lu-PSMA-617. The treatment itself took a few hours, during which he was monitored in a specialized nuclear medicine unit. Subsequently, post-treatment scans confirmed the radiation had reached all the targeted lesions, including his spinal metastases that were causing him considerable pain.
Step 3: Managing Side Effects. Over the next few days, John experienced mild fatigue and temporary dry mouth, which were managed with hydration and salivary gland stimulation techniques. His blood counts dropped slightly but recovered naturally before the next cycle.
Step 4: The Outcome. After the third cycle, a follow-up PSA blood test showed a significant drop in his Prostate-Specific Antigen levels—a key indicator of cancer activity. A repeat Ga-68 PSMA PET/CT scan four months later showed a remarkable reduction in the size and metabolic activity of his metastatic tumors. Therefore, Lu-177 offered John a new lease on life, demonstrating the powerful potential of this targeted approach.
Who is This For? Determining Patient Eligibility
While Theranostics treatment with Lu177 for metastasis is revolutionary, it is not a universally applicable therapy. Patient selection is rigorous and highly dependent on the specific type of metastatic cancer. Cancer treatment cost and eligibility are often closely linked to the diagnostic stage, which is crucial here.
The Core Principle: The Target Must Be Present.
Essentially, the treatment is reserved for patients who meet stringent criteria:
- Metastatic Castration-Resistant Prostate Cancer (mCRPC): Patients must have failed prior standard treatments, including taxane-based chemotherapy and novel anti-androgen agents. Crucially, they must demonstrate sufficient PSMA uptake on a diagnostic PSMA PET scan.
- Neuroendocrine Tumors (NETs): Patients must have well-differentiated, inoperable, or metastatic NETs that are progressing or symptomatic. The diagnostic criteria require high expression of SSTR2 receptors on a Ga-68 DOTATATE PET/CT scan.
- Adequate Organ Function: Patients must have sufficient kidney function (because of the clearance process) and bone marrow reserve (to withstand temporary suppression).
- Performance Status: Patients must generally be well enough to tolerate the multi-cycle treatment regimen.
Thus, if you or a loved one are facing advanced prostate cancer or NETs, consulting with a specialized nuclear medicine oncologist is the necessary first step to determine theranostics eligibility.
Comparison Table: Lu-177 Theranostics vs. Traditional Systemic Therapy
To highlight why Theranostics treatment with Lu177 for metastasis is gaining prominence, compare its profile against traditional systemic treatments like chemotherapy.
| Feature | Lu-177 Theranostics (PSMA/PRRT) | Traditional Chemotherapy |
|---|---|---|
| Mechanism of Action | Internal, targeted delivery of low-range beta radiation directly to the tumor cell receptor. | Systemic distribution of cytotoxic drugs that kill rapidly dividing cells (both cancerous and healthy). |
| Specificity | Extremely high, relying on unique tumor surface markers (PSMA/SSTR2). | Low, relies primarily on the cancer cells’ high division rate. |
| Primary Side Effects | Dry mouth (PSMA), temporary blood count drops, fatigue, potential kidney effects (PRRT). | Nausea, vomiting, hair loss, severe fatigue, high risk of infection (due to severe myelosuppression). |
| Treatment Delivery | Usually 4-6 cycles, several weeks apart. Relatively brief infusion/injection time. | Repeated cycles every 2-3 weeks, often requiring long infusion times and aggressive symptom management. |
| Diagnostic Requirement | MANDATORY diagnostic scan (PSMA or DOTATATE PET) to confirm target presence. | Not required; administered based on tumor type and general clinical stage. |
Safety, Side Effects, and Future Outlook
Understanding the safety profile is crucial for anyone considering Theranostics treatment with Lu177 for metastasis. While highly targeted, the radioisotope does circulate throughout the body, interacting with normal organs to some degree. Consequently, careful monitoring is the cornerstone of this treatment.
Monitoring and Management
The two main organs requiring vigilance are the bone marrow and the kidneys. Every patient undergoes frequent blood tests, especially to check for myelosuppression (low blood counts). Treatment for other cancers is often more harsh, but with Lu-177, the side effects are often temporary. If bone marrow suppression is detected, the next treatment cycle may be delayed or the dose adjusted. For PRRT, the amino acid infusion prevents significant kidney damage. Furthermore, continuous surveillance is key to ensuring long-term safety.
The Future of Lutetium-177 Theranostics
The success of Lu-177 in prostate cancer and NETs is merely the beginning. In addition to these established uses, researchers are actively exploring new targeting molecules to apply Lu-177 to other metastatic cancers, including:
New molecules targeting other tumor types are in development.
Specifically, clinical trials are investigating Lu-177 conjugates targeting:
Emerging research suggests application potential in breast and lung cancers.
- FAPI (Fibroblast Activation Protein Inhibitor): Targeting FAPI-positive tumors, which include sarcomas and difficult-to-treat forms of lung cancer and gastrointestinal cancers.
- Melanoma Targets: Investigating targets for metastatic melanoma where other therapies have plateaued.
Thus, the potential for expanding Theranostics treatment with Lu177 for metastasis across a broader range of solid tumors is immense, promising a more targeted and less debilitating future for cancer care. We are committed to keeping up-to-date with new methods in IVF and oncology, which are driven by similar precision principles.
Moreover, international collaboration between institutions is driving rapid innovation, sharing data on long-term outcomes and safety protocols. For example, major university centers are publishing findings that inform best practices worldwide:
Read a recent paper on Lu-177 efficacy published by Harvard researchers.
Addressing Common Concerns: Outbound Research Links and Patient Education
For patients and professionals seeking deeper knowledge, we strongly recommend reviewing primary research from academic leaders in the field. These studies solidify the clinical evidence behind Theranostics treatment with Lu177 for metastasis and its role in advanced disease management.
Notable Research Sources (Normal Links):
- For an authoritative overview of the theranostics mechanism:
View the University of Chicago’s latest trial results for Lutetium NETs. - On the long-term safety profile of PRRT for neuroendocrine tumors:
Duke University’s comprehensive review on molecular imaging and theranostics. - For a deeper dive into PSMA-ligand development and optimization:
Weill Cornell Medicine’s research on next-generation Lu-177 molecular therapy. - Discussing the integration of Lu-177 with other systemic therapies:
Johns Hopkins Medicine’s overview of theranostics integration in oncology. - Evaluating comparative effectiveness against second-line chemotherapies:
UCSF Health’s guide on PSMA PET and PRRT for prostate cancer treatment.
For further clinical data and safety reporting (Nofollow Links):
It’s important to consult various institutional data, particularly concerning potential side effects and specialized patient management protocols, especially if considering medical travel for treatment. Reviewing institutional protocols provides confidence and clarity regarding the level of care.
- Information on patient selection criteria and pre-treatment screening:
Yale Cancer Center’s latest data on Lu-177 safety and efficacy. - Detailed protocols for minimizing renal and salivary gland toxicity:
Cambridge University’s research on nuclear oncology in metastasis. - Review of clinical outcomes for complex metastatic cases:
Oxford University’s perspective on the future of nuclear medicine. - Expert consensus on dosing and treatment cycle frequency:
Massachusetts General Hospital’s protocol insights for PSMA-Lu177 therapy. - Guidelines for post-treatment monitoring and follow-up care:
MD Anderson Cancer Center’s expert insights on PSMA PRRT.
Ultimately, the integration of Theranostics treatment with Lu177 for metastasis into clinical practice relies on this continuous research and adherence to strict protocols.
Frequently Asked Questions (FAQ) about Lu-177 Theranostics
These answers provide clear, non-interactive summaries of the most common questions surrounding this advanced treatment.
1. What exactly is the difference between PSMA and DOTATATE therapies?
The difference lies in the target: PSMA therapy uses a molecule to target the Prostate-Specific Membrane Antigen found on prostate cancer cells. In contrast, DOTATATE therapy (PRRT) targets the Somatostatin Receptors (SSTR2) found on neuroendocrine tumor (NET) cells. Both, however, utilize the Lu-177 radioisotope for the therapeutic dose.
2. Is Lu-177 Theranostics considered a cure for metastatic cancer?
Currently, Lu-177 theranostics is generally considered a highly effective, life-prolonging, and symptom-controlling treatment for advanced, metastatic disease, rather than a definitive cure. It often leads to significant disease stabilization, tumor shrinkage, and improved quality of life, but further cancer treatment may be necessary over time.
3. How long does the radioactive effect of Lu-177 last in the body?
Lutetium-177 has a half-life of 6.7 days. This means that half of the radioactivity decays every 6.7 days. The effective biological half-life (how long it stays in the body) is shorter, as the drug is quickly eliminated. Most patients are safe to return to normal interactions within a few days to a week after treatment, following specific institutional radiation safety guidelines.
4. Can Lu-177 Theranostics be used alongside chemotherapy?
It is generally not administered at the exact same time as chemotherapy due to the potential for overlapping bone marrow suppression. Treatment typically follows a failure of standard chemotherapy or is used in combination sequences rather than simultaneous administration. This integration is part of the broader oncology guide to sequenced therapies.
5. What is the typical treatment schedule?
The standard regimen usually involves 4 to 6 cycles, with each cycle separated by 6 to 12 weeks. This interval allows the bone marrow and other organs to recover from the localized radiation exposure before the next dose is administered.
6. Do I need to be isolated after the treatment?
Yes, but typically only for a short period. Due to the lingering radioactivity, patients are usually isolated in a specially shielded room for 24 to 48 hours immediately following the infusion. After discharge, restrictions generally involve minimizing prolonged close contact with others, especially children and pregnants, for about a week.
7. What if my scan shows that the tumor doesn’t have the target receptor?
If the diagnostic scan (Ga-68 PSMA or DOTATATE PET/CT) shows insufficient uptake, the patient is ineligible for Lu-177 theranostics because the treatment wouldn’t reach the tumor cells effectively. In such cases, the oncologist would pivot to other advanced options, such as TIL therapy or CAR T-cell therapy, depending on the cancer type.
8. Is this treatment covered by insurance?
Coverage varies significantly by country, healthcare system, and insurance provider. As this is an advanced, high-cost therapy, pre-authorization is almost always required. Patients often consult a global medical tourism guide if local access or coverage is difficult.
9. Can Lu-177 be used for bone metastases?
Absolutely. Lu-177 theranostics is exceptionally effective against bone metastases, provided the lesions express the necessary target (PSMA for prostate cancer or SSTR2 for NETs). The localized radiation not only shrinks the metastatic tumors but often provides significant pain relief from the bone lesions.
10. What is the preparation process before a cycle of Lu-177?
Preparation typically involves strict hydration, stopping certain medications temporarily, and ensuring recent blood tests confirm adequate organ and bone marrow function. For PRRT, the kidney-protective amino acid infusion is started before the Lu-177 administration. This mirrors the necessary checks for procedures like gynecological surgery or pre-travel resources planning.
11. Are there any dietary restrictions during or after treatment?
Generally, there are no strict long-term dietary restrictions. Short-term, patients may be advised to maintain high fluid intake to help flush the radioisotope from the body and may need to adjust to managing dry mouth if it occurs. However, patients facing gastrointestinal cancers may have separate dietary needs.
12. How does the Lu-177 isotope compare to other radioisotopes like Iodine-131?
Iodine-131 (I-131) emits higher-energy beta and gamma rays, traveling further in tissue, which is useful for treating large tumors like thyroid cancer but can cause more collateral damage. Lu-177’s lower energy and shorter path length make it superior for highly targeted treatments like PSMA and DOTATATE, where precision is paramount, minimizing harm to neighboring healthy cells.
13. What happens if the metastatic cancer is from breast or lung origin?
Currently, the standard Lu-177 treatments (PSMA and DOTATATE) are not approved for most breast or lung cancers, as these tumors typically do not express PSMA or SSTR2. However, research is ongoing with new targeting ligands for other tumor types, like FAPI, which shows promise for certain aggressive lung and thoracic tumors.
14. What long-term follow-up is required after completing the full Lu-177 regimen?
Long-term follow-up involves periodic blood tests (e.g., PSA for prostate cancer, Chromogranin A for NETs), frequent imaging (CT or MRI), and routine kidney and bone marrow function checks. This monitoring ensures that the disease remains stable and that any potential long-term side effects are promptly managed. This is key to ensuring continuous care, similar to the long-term management required after complex procedures like hair transplant results.