Description
🧬 CAR-T Cell Therapy: The Ultimate Guide to Cancer’s New Frontier 🛡️
SKU: WMT-CAR-T-2025
Executive Summary: T-Cells Unleashed
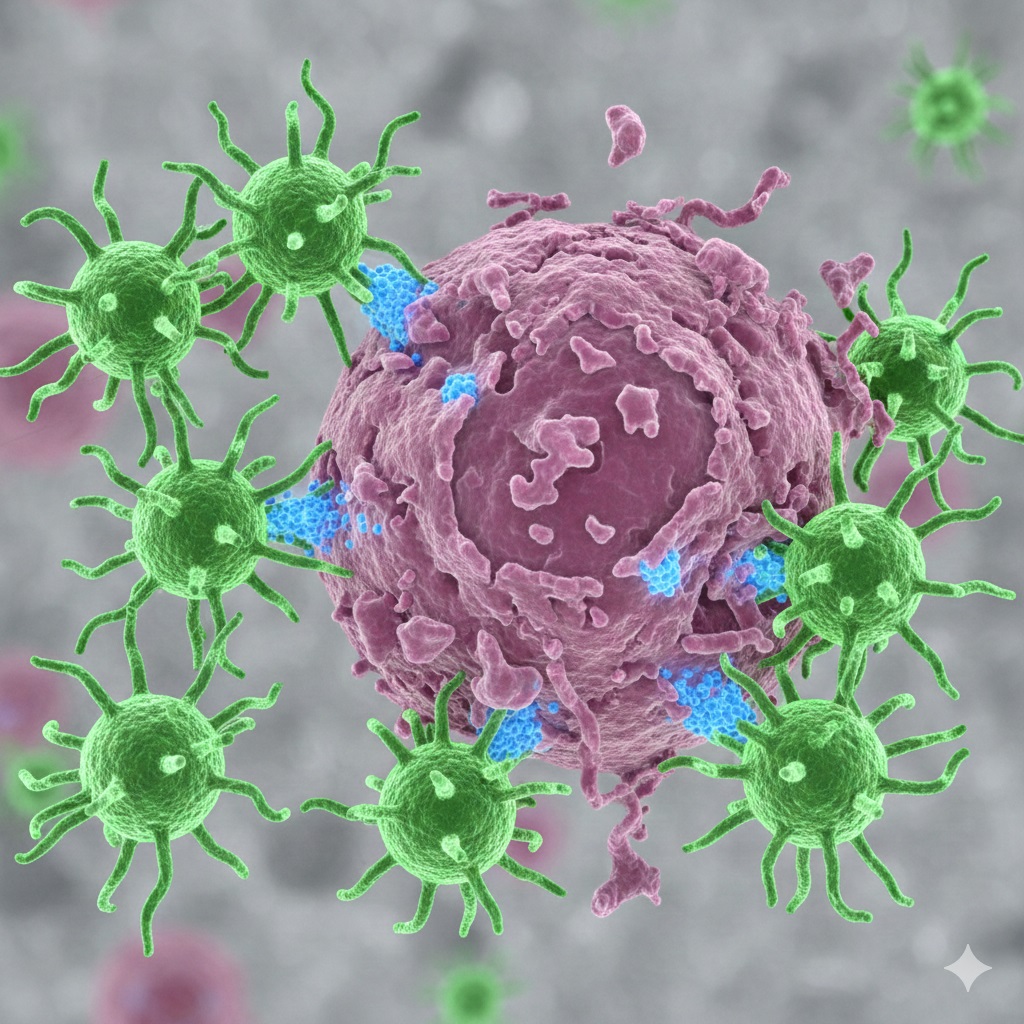
CAR-T Cell Therapy represents a monumental leap in personalized cancer treatment. Instead of relying on conventional methods like chemotherapy or radiation, this innovative therapy engineers a patient’s own immune cells—T cells—to become specialized cancer-fighters. This process involves collecting T-cells, modifying them in a lab with a Chimeric Antigen Receptor (CAR), and reinfusing them. The re-engineered cells then seek out and destroy cancer cells with remarkable precision. Also, **CAR-T Cell Therapy** is sometimes referred to as a type of cell-based gene therapy.
Key Takeaways:
- Personalized: Uses the patient’s own immune cells, genetically altered to attack specific cancer antigens.
- Effective: Offers deep, durable remission for specific blood cancers, including Leukemia, Lymphoma, and Multiple Myeloma.
- Advanced: This is a complex, multi-stage process that typically takes 4–5 weeks and requires expert medical centers.
- Global Access: Wmedtour helps you access leading oncology departments worldwide for this advanced care.
What is CAR-T Cell Therapy? (Chimeric Antigen Receptor T-cell Therapy)
CAR-T Cell Therapy is an advanced form of immunotherapy and cell-based gene therapy. Essentially, it transforms your body’s T-cells—a type of white blood cell and a key component of the immune system—into ‘smart bombs’ specifically programmed to recognize and attack cancer. Importantly, cancer cells often possess surface antigens that the natural immune system overlooks. The Chimeric Antigen Receptor (CAR) gives the T-cell a new receptor so it can bind to those specific cancer antigens and eliminate the threat. Because the CAR is engineered to target a specific antigen, a CAR-T cell therapy made for one type of cancer will not work against another type.
This groundbreaking approach currently shows the greatest success against hematological malignancies, including certain types of Acute Lymphoblastic Leukemia, non-Hodgkin Lymphoma, and Multiple Myeloma. Moreover, this therapeutic coverage is expanding, with successful treatments extending to certain Digestive System Tumors, such as gastric cancer/gastroesophageal junction adenocarcinoma, and pancreatic cancer. Furthermore, researchers aggressively explore its application for solid tumors, continually expanding the scope of what this treatment can achieve.
🔬 Comprehensive Targets and Wide Disease Coverage
To fully appreciate the precision of this treatment, it is vital to understand the comprehensive targets successfully covered by **CAR-T Cell Therapy**. Consequently, the therapy is categorized by the specific antigen it is designed to bind to:
Targeted Antigens for Immunotherapy:
- Hematologic Targets (Single): CD19, CD20, CD22, CD7, CD5, BCMA, GPRC5D, CLL1, and others.
- Hematologic Targets (Dual): These sequential CAR-T approaches, such as CD19-22 or CD19-20, offer an advanced strategy to prevent tumor escape.
- Solid Tumor Targets: New targets, including Claudin18.2 CAR-T, are driving the promising expansion into solid tumors.
Diseases Successfully Covered by CAR-T:
- Hematologic Tumors: Leukemia, Lymphoma, and Myeloma.
- Digestive System Tumors: Gastric cancer/gastroesophageal junction adenocarcinoma, pancreatic cancer, and others.
- Other Conditions: Initial research also explores its application for Autoimmune Diseases.
If you are exploring other advanced treatments, consider reading our guide on new methods in cancer treatment.
💡 The Personalized CAR-T Therapy Process
The journey through CAR-T Cell Therapy is comprehensive, typically spanning 4–5 weeks. Consequently, proper planning and coordination are paramount. Here is a step-by-step breakdown of this complex, highly personalized procedure:
Stage 1: Assessment and Collection
First, patients need to undergo a rigorous pre-treatment assessment (3–5 days), including comprehensive evaluations, imaging tests, laboratory tests, functional assessments, and a bone marrow biopsy. Once cleared, doctors proceed with T-Cell Collection. During this step, a process called apheresis draws blood from the patient to collect white blood cells. This collection takes approximately 3–5 hours.
Stage 2: Gene Engineering and Manufacturing (The CAR-T Cell Factory)
Next, the collected apheresis product is sent to a specialized laboratory. This is where the scientists genetically engineer the T-cells to express the Chimeric Antigen Receptor (CAR). This engineering phase is critical. They culture the new CAR-T Cells in a lab until they reach the required millions of doses. Manufacturing typically requires 6–8 days. This revolutionary method truly embodies personalized medicine.
Experts manage this process to ensure the cells are prepared correctly. Read about other innovative T-cell therapies in our TCR-T Cell Therapy guide.
Stage 3: Infusion and Monitoring
The facility administers appropriate bridging therapy and lymphodepleting chemotherapy (3 days) to reduce the tumor burden in patients before the infusion. This essential step, therefore, creates space for the new, powerful **CAR-T Cells** to expand and function effectively. The CAR-T Cell Therapy infusion itself is straightforward, similar to a blood transfusion, lasting only 10–20 minutes to complete. Subsequently, patients enter a crucial monitoring phase, staying in the hospital for 15–30 days. This close observation allows the medical team to promptly manage potential side effects and adverse reactions.
🤝 Comprehensive Management and Combination Strategies
Given the complexity of **CAR-T Cell Therapy**, a robust management and care framework is indispensable. For this reason, treatment is handled by a holistic approach:
Comprehensive Management Services:
This process is carefully managed to ensure patient safety during the entire treatment. The management services involve the meticulous assessment, implementation, monitoring, and care of the therapy.
Multidisciplinary Care:
The best outcomes result from collaborative care. Medical teams collaborate with disciplines such as integrated diagnostics, infectious imaging, and respiratory medicine. This specialized cooperation forms distinctive, interdisciplinary fields essential for navigating the potential complications of the therapy.
Combination Strategies:
Finally, a critical development is the combined application of **CAR-T Cell Therapy** with traditional methods. These strategies integrate CAR-T with existing treatments, such as chemotherapy, targeted therapy, and even hematopoietic stem cell transplantation, to enhance efficacy and prevent relapse. This forward-looking approach is defining the next generation of cancer care.
CAR-T: Advantages and Critical Considerations
✅ Pros (Advantages of CAR-T Cell Therapy)
- High Efficacy: Achieves deep, long-lasting remission in certain relapsed/refractory blood cancers where other treatments have failed.
- Precision: Targets cancer cells with incredible specificity, minimizing damage to healthy tissues.
- A Single Treatment: Often provides therapeutic benefit after a single infusion, unlike long courses of chemotherapy.
❌ Cons (Critical Considerations of CAR-T Cell Therapy)
- Side Effects: Can cause severe, but manageable, side effects like Cytokine Release Syndrome (CRS) and neurotoxicity.
- Cost: The expense remains very high globally, making medical tourism a necessity for many. See our cancer treatment cost by country guide.
- Availability: It is only approved for specific cancer types and is only available in highly specialized, certified medical centers.
Comparison Table: CAR-T vs. Traditional Cancer Treatments
To better understand the distinct role of **CAR-T Cell Therapy**, compare it to more established cancer treatments. This table illuminates why CAR-T is often a choice for late-stage or refractory disease:
| Feature | CAR-T Cell Therapy | Standard Chemotherapy | Targeted Therapy (Small Molecule) |
|---|---|---|---|
| Mechanism | Personalized, living drug (re-engineered T-cells). | Chemical agents destroy rapidly dividing cells. | Drugs block specific pathways necessary for cancer cell growth. |
| Specificity | Extremely high (targets specific antigen). | Low (targets all fast-dividing cells). | High (targets one specific protein/receptor). |
| Treated Cancers | Select blood cancers (Leukemia, Lymphoma, Myeloma) and certain solid tumors. | Wide range of blood and solid tumors. | Specific cancers with known genetic markers. |




Reviews
There are no reviews yet.